- Ben Straub (GSK)
- Dadong Zhang (Illumina)
- Eva Li
- Heidi Curinckx (Johnson & Johnson)
- HyeSoo Cho (FDA)
- Joseph Rickert (R Consortium)
- Margaret Wishart
- Nan Xiao (Merck)
- Ning Leng (Roche/Genentech)
- Paul Schuette (FDA)
- Robert Devine (Johnson & Johnson)
- Saghir Bashir (Argenx)
- Sam Parmar (Pfizer)
- Steven Hasendinckx (Johnson & Johnson)
- Youn Kyeong Chang
The meeting was recorded and the video is available.
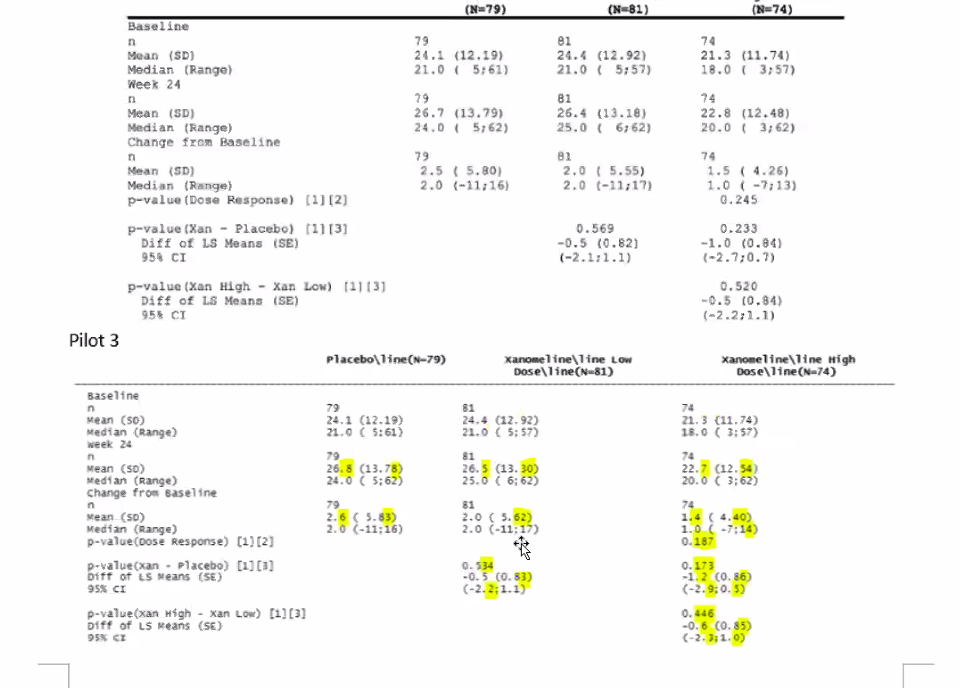
Pilot 3
HyeSoo presented an update on the continuing FDA evaluation of Pilot 3.
Using the R generated ADaM data sets, the FDA reviewers were able to replicate the results of the demographic, efficacy, primary and KM plot analyses
The reviewers compared the results of the four analyses between Pilot 1 and Pilot 3.
- Primary output in Pilot 3 was different from the Pilot 1 result due to the QC findings
- QC findings in ADRG include all of the discrepancies between the original ADaM datasets
- The discrepancies in ADADAS data sets cause different primary analyses outputs
- This is an issue from the CDISC ADADAS
- It is not clear which CDISC file, the file from Pilot 1 or the file from Pilot 3, is correct.
The following figure compares the Pilot 1 and Pilot 3 primary output. Discrepancies are highlighted.
 Additional FDA questions included:
Additional FDA questions included:
- Why is there an indication in the output of LOCF, “Last observation carried forward” when the original data set had no missing data?
- There was no statistical analysis plan included in the submission.
- Action: Ben Straub will confer with the Pilot 3 team to answer these questions and review the discrepancy problems between Pilots 1 and 3.
- Action: Paul and HyeSoo will discuss whether we need to resubmit Pilot 3 The next meeting of the working group will be on Friday, February 2, 2024 at 9AM Pacific Time.
- All agreed that if a resubmission of Pilot 3 is required, we will consider submitting via a .zip file.
There was some discussion about what we could do to improve the quality control of our submissions.
- The group agreed that we should have a check list to compare items against a Pilot 1 source of truth.
- We should have additional reviewers who are not close to the work.
- The idea of using AI tools to check consistency was mentioned.
Upcoming Webinar
On Monday at 7PM PST Rikimaru Nishimura and Yuki Matsunaga will present a webinar on The Adoption of R in Japan’s Pharma Industry Confirmation. Look here for details and information on how to register.
- Ning noted that Yuki expressed an interest in collaborating with the R Consortium.
- Action: we will set up a meeting with the Japanese team after the meeting.
PharmaSUG
- JBR mentioned that it is an R Consortium goal for 2024 to make the opportunity for as many speaking engagements as possible.
- Ben mentioned that PharmaSUG 2024 is coming up in May,
- Action: Ben volunteered to submit an abstract by the January 15 deadline. Ning will provide Ben with some copy used for a previous abstract.
The next meeting of the working group will be at 9AM PST on Friday, February 2, 2024.